The Creative Science Centre
Picture Gallery
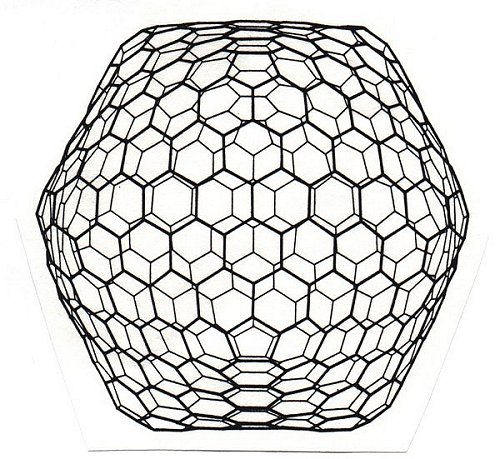
Giant Fullerene with 540 atoms, 12 pentagons, 260 hexagons
A general rule for the number of atoms (n) in such a large fullerene is to count the number of bonds (a) that lie between the corners of the pentagons to the next pentagon (in this case a=3) square this number and times by 60 (i.e. n=60xaxa, 3x3x60=540 atoms). The number of bonds (b) is 1.5 times the number of atoms (i.e. b=1.5xn, 1.5 x 540 = 810 bonds). The number of hexagons (h) is give by h=(n-20)/2=(540-20)/2=260 hexagons.
(picture, Ken McKay, The Sussex Fullerene Centre)
THE CREATIVE SCIENCE CENTRE
Dr Jonathan Hare, Room 3R253, Chichester Bldg. CPES, The University of Sussex
Brighton, East Sussex. BN1 9QJ. 01273 606755 x3171
home | diary | whats on | CSC summary | latest news