
Figure 1. two of the many possible circuit arrangements for making a battery from 12 sea water cells
Summary
Simple homemade batteries are described that can be made from items found in the home or school lab. These experiments help students/pupils understand how to wire them up, the origin of current, voltage and power, as well as of course the chemistry that drives the batteries. There is ample scope here for school/home project investigations, experimentation and innovation.
Introduction
The modern battery is found in high-tech applications ranging from hearing aids and pace-makers to pocket calculators, personal stereos, radios and mobile phones. They are everywhere and completely invaluable. The origin of the power behind the battery is chemical. In the following article we explore some simple experiments involving homemade sea- water batteries that are not only instructive and fun to make, but which can be used to drive low-power devices.
The simplest cell (a battery is simply a group of cells) is made by putting two different metal electrodes into an electrolyte (usually a solution of a salt or acid in water). Each metal receives an electrical charge and one becomes more positive (+) than the other (-). Because opposites attract, there is an energy of attraction (called the voltage) between the charges, which is the origin of the battery's power (see below for a more full explanation of voltage, current, energy and power) of the battery.
A simple battery can be made by pushing a zinc-coated screw and a piece of copper into a lemon. This wonderfully simple cell can even produce enough power to run a LCD clock / watch and its worth experimenting with the cheap 'toy' watchs that are easy to find in markets. A carbon-rod electrode instead of the copper will also work. Three potatoes each having a carbon rod and a zinc-coated screw electrode system can be wired in series (like a daisy chain) to power an LED (Light Emitting Diode) light. Here we describe carbon rod and screw batteries using sea (salt) water as an electrolyte, which are made from household ice cube trays!
How they batteries work - electrolysis
The voltage created in a battery is due to ionic chemistry. When a metal electrode is immersed in an electrolyte a rather complex dynamic process occurs. Let us assume that the metal electrode is initially uncharged (the atoms from which the electrode is made are neutral - they have equal numbers of electrons and protons). When the metal is immersed in the electrolyte, positive metal ions are formed on the surface of the electrode. These ions pass into solution, making the electrode progressively more negative, as the positive ions move away. There will come a time, however, when the negatively charged electrode will start to attract back the oppositely charged ions. So a dynamic equilibrium is thus formed between those ions leaving and those returning to the metal surface. How far the equilibrium goes one way or the other is dependant on the reactivity of the metal.
A battery must have two electrodes, so meanwhile on the other electrode, a similar process must be taking place. If this second electrode is of the same metal as the first, then each electrode will charge to the same extent (voltage), and there will be no resulting difference between them. There will thus be no attractive force (electromotive force EMF) between them, and so no current will flow.
However, if the electrodes are of different metals, then their reactivity's will be different, and so different equilibria will be set up. One electrode will become charged to a greater extent than the other and because of the difference in reactivities will have a different voltage. In other words, there will now be a voltage difference between the electrodes. Because of this difference, electrons will want to move from one electrode to the other. This attractive potential is the voltage that we measure between the electrodes, and the origin of the batteries electrical power.
The Electrochemical Series
If we imagine a series of batteries, each made up of one metal electrode but each also having a 'standard electrode' (the standard that is used is an electrode composed of a platinum wire in a hydrogen gas envelope but we don't need to know much of the details here). We can draw up a table, called the Electrochemical Series, of the voltages arising from these different batteries. Such a table will be useful for comparing different electrode systems. We can actually use it to predict the voltage of a cell made up from two different metals (i.e. without the reference electrode). It is simply the difference between the two values of the standard electrode potentials (Note: it is important to keep in mind the sign + / - of these standard potentials when doing the calculation).
Voltage, current and power
As opposites attract, once the electrical circuit is completed, there will be a tendency for the negative electrons to be attracted to the more positively charged electrode. If a wire is connected between the two electrodes, this is precisely what happens. This potential energy (work that can be done) to move electrons when a circuit is conected is called the voltage of the battery:
V = Volts = energy per charge (joules / coulomb).
The amount of charge passing per unit time is known as the current:
A = Amp = rate of charge passing (coulombs / sec).
If we multiply the voltage between the electrodes by the particular current that is passing, we get the power of the cell:
P = Power = V x A = (joules / coulomb) x (coulombs / sec) = joules / sec
Which is the work done per time.
Battery efficiency
The voltage is dependant on the difference between the individual electrode potentials. However, in practice, this is only true in the ideal case where we can measure the voltage without drawing any appreciable current. This is the case when using a very high resistance voltmeter, but not the case when we use the battery to drive a radio, for example. In practice, what we find is that, as we start to draw current from the cell the voltage drops away.
This limitation of a real life battery is due to at least two factors; the nature of the electrolyte and the electrical resistance of the electrodes. The dependency of the battery voltage on the electrode potentials explains why similar results can be obtained using different electrolytes (eg.for example: Salt water, vinegar, sulphuric sulfuric acid, and even urine!). But of course the battery must also be dependant on the concentration and type of ions in the electrolyte. The limiting case being when there are no ions present at all (e.g. in a non ionic liquid or a battery that has dried out etc.).
Assuming that the electrolyte is above a threshold concentration the surface resistance of the electrodes then has to be considered. This is the electrical resistance made between the solid electrode and the solution due to the flow of electrons and ions. If the electrode resistances are low, the cell voltage will drop of slowly with rising current. If the resistance is high, the voltage will appear to drop as current is drawn from the battery. The internal resistance of cells is a major limiting factor in the application and usefulness of a real battery.
An experimental sea sea-water battery
We have seen how a pair of electrodes can produce a voltage when immersed in an electrolyte. In the following experiments we use of galvanised (zinc- plated) screws and carbon rods as the electrodes and salt water as the electrolyte. While carbon is a good conductor of electricity, the chemistry that takes place at the carbon electrode is more complicated than would be the case when simply using metals. However, larger potentials are produced with carbon and zinc than with copper and zinc, so it is worth the complication. Carbon is a good conductor of electricity. In these cells the metal (zinc) electrode is negative (-) while the carbon becomes positive (+).
To get round the limited voltage and current of such a simple cell, we can join up cells to make a battery of cells - thereby increasing the power. An effective arrangement is shown in the diagram Figure 1. Household ice- cube trays are used to hold the electrolyte, and wood supports the multiple pairs of electrodes, a set for each ice cube tray.
Making the battery
Each of the ice cube trays is 3/4 filled with a salt solution (sea water or a solution of table salt in water). Galvanised screws can be purchased from any hardware store. Pencil leads can be used for the carbon rods or, better still, they can be salvaged from carefully dismantled old ('flat') batteries. Then the electrode pairs are lowered into their respective ice cube tray solutions to create the 12 cells. They are then wired-up on the top side of the wooden support to form the battery.
Wiring the the cells up in series or parallel?
So what is the best way to wire up the 12 cells to get useful power from the battery? Consider a single cell; it can produce a voltage of V volts and a maximum current of say I amps. Wiring a number (n) of these cells in series (one after the other in a sort of daisy chain) will multiply the voltage giving n x V volts. However, the maximum current produced by this arrangement will be the same as that of a single cell - I. On the other hand wiring all the cells in parallel will increase the curent n-fold but maintain the voltage equivalent to that of a single cell (i.e. V). Combinations of series and parallel cells with produce combination of possible total V and I.
The ice cube tray used in these experiments had 12 compartments (ice cubes) and so to get useful power from the battery two combinations of wiring were chosen (see Figure 2): The first a) consists of two sets of six cells wired in series and these two sets then wired in parallel - giving a total of 6 x V and 2 x I. b) consisted of two sets of six parallel parallel cells which were wired in series - giving a total of 2 x V and 6 x I.

Parts list:
Table 1) Salt (NaCl)
2) Ice- cube trays
3) Wood for electrode support
4) galvanised screws ( ca. 5 cm long) for each battery
5) 12 pencil leads (2B or softer), or better still, school lab carbon rods or ones salvaged from old worn out batteries
6) Tinned copper wire

A sea water power plant (!)
The first battery circuit provides a relatively higher voltage than the second and so it can therefore be used to power devises devices that need 'higher voltages' but low currents. A pocket LCD calculator, an LED (and series resistor), and possibly a pocket radio, will work well using this arrangement. In the demonstrations we use a simple flashing LED circuit to dramatically show the battery working. This circuit requires about 3V, but only about 1 or 2 mA to work.
Please Note: remember to check that the device you are powering is correctly wired to the – and + connection of the battery (metal (zinc) = -, carbon = +).

The second battery circuit will work well on for devices that need a greater current but not a particularly high voltage. A good example of this would be a low voltage motor. Some motors will run on only about 1 volt but need 10 mA or so in order to turn (see notes section below for details of suitable motors).
Ideas for further experiments
The basic battery described above is capable of driving low power devices. As the battery is a device that converts chemical potential into electrical potential eventually the battery will fail (run down) as the chemistry develops at the electrodes and in the electrolyte. The zinc on the screws is dissolving and their may also be zinc hydroxide (and / or zinc chloride) forming on the electrodes which will increase the resistance. The electrodes will therefore need to be cleaned before each use of the battery to reduce the resistance and the chemical build up that inevitably occurs.
The obvious places for further experimentation are to try different electrode materials (what is the effect of the surface area of the electrodes for example) and try using different electrolytes (for example try orange juice, vinegar, sulphuric sulfuric acid, or even urine!). Does the current produced from the battery simply depend on electrolyte concentration or does it fail at some threshold value? What is the effect of the temperature of the electrolyte in the cells and if so why does it have an effect? Does this help to explain why you can rejuvenate used batteries by putting them on a warm radiator?
If you want the battery to start to work (to be activated) when immersed in sea water then try putting small pieces of sponge between the electrodes. When the empty battery is immersed, the sponges soak up sea water so that electrolyte remains between the electrodes when the battery is taken out. (If you leave the contraption in the sea many of the cells would be shorted out by the sea water - hence you need to take the battery out of the sea after the water gets in). This was the way we made the emergency life jacket lights in the fifth series of Rough Science set in Zanzibar (BBC2 Feb. 2005). When the life jacket went into the sea it activated the battery, powering the emergency lights.
The Baghdad Battery
In 1800 Count Alessandro Volta made what was thought to be the first device that we can consider as a modern day battery. He found from experiments that different metals in contact with each other via salt solution soaked strips created electricity. He made a device composed of an alternating pile of metal coins sandwiched between electrolyte (salt water) moistened felt strips. Connection to the two end of the pile allowed access to the voltage. This device became known as a 'voltaic pile' (historically this is where the terms volt and voltages got there names). In their modern form batteries are a recent invention although of course voltages have been present since the elements were formed and electrolytes could form and also in creatures such as electric eels etc. But was Volta's pile the first battery? There is however a rather controversial theory that batteries might have been around for more than 2000 years!
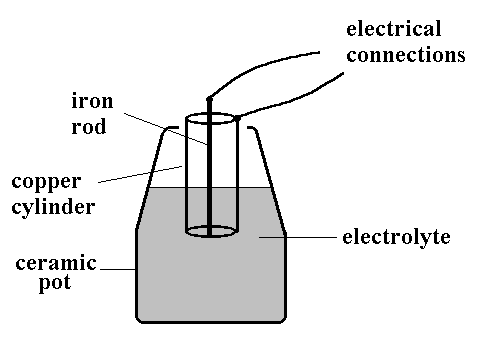
In 1938 an archaeologist Von Wilhelm Konig working at the Baghdad Iraq Museum, claimed to have discovered a very old battery. He thought the Mesopotamians had invented the battery in 200 BC! ! His so-called 'Baghdad Battery', shown in the picture, consisted of a ceramic pot about 20cm high, in which was placed a cylinder of copper metal and an iron rod. It appears that the iron rod was surrounded by an oil/pitch based insulator, and evidence for organic material was found in the pot.
Recently we have made copies of these devices using vinegar (which of course would have been an available electrolyte at the time). A single device produces about 1V at 10mA. Of course from what we have learnt in this article such a system would indeed work as a battery, but was this the intention over 2000 years ago - did the Mesopotamians really mean to invent the battery, or did the device have some other, quite unconnected purpose?
It seems very un-likely that they purposely made a battery, especially as no wires were found with the device. However, assuming that they were using them as batteries, there are several theories that have been put forward for their purpose? One suggestion was that the Mesopotamians may have wired up many of the batteries so that they could electrify holy statues to give them 'magical' powers. This is a nice theory / idea perhaps, but electric shocks depend on high voltage, and they would have needed hundreds of batteries to produce a shock!
Another theory is that these small voltages could have been used in acupuncture (which had been discovered / invented by this time). Some of the devices batteries were found next to fine needles. It is possible that needles put in the skin and wired to a few volts might have produced therapeutic effects - well, some kind of effect anyway!
Finally in the course of our own recent investigations into Baghdad batteries, we tried to use the voltage from several batteries in series to drive a simple electrolysis reaction. The idea was to produce silver ions from a silver electrode (we used a silver bracelet), and use it to plate another (cheaper) metal electrode. Using silver and copper electrodes in a vinegar electrolyte we found (given enough time) that we could successfully silver (although not very nicely!) plate the copper using our set-up!
So, could the ancient Mesopotamians have used the similar Baghdad batteries to plate cheaper intricately designed copper statues with a silver layer? Unfortunately, no proof for this wonderfully appealing possibility exists in the archaeological records. The origin, use and purpose of the so called Baghdad batteries therefore still remains a mystery.
References
talks and workshops
For details of the electrochemical series click here
For news articles about the Baghdad Batteries click here
Notes
1) We have found that 'carbon rods' are much better than 'graphite rods' of the same purity. Even though both types may be high purity carbon the 'carbon' rods appear to be more porous than the shinny graphite rods. The carbon rods therefore present a much greater surface area to the electrolyte. As a consequence the electrode resistance is much less, creating a better battery.
2) We made our first sea-water battery on in the very first series of Rough Science, and we have been hooked ever since! The batteries described here are regularly used in an electricity workshop we run at the CSC. In the fifth series of Rough Science (Zanzibar, Feb. 2005, BBC2) we used sea water batteries to power emergency lights on a life jacket.
3) It is worth checking the various companies that sell electronic component as most stock 'ultra bright' LED's as well as 'solar-cell' motors that work on very low voltages and currents suitable to be powered by these experimental batteries.
Acknowledgments
We would like to thank NESTA, the BBC and the Open University. In particular: David Shulman, Paul Manners, Mile Lehey and all the Rough Scientists (of all ages and countries). Many thanks to Graham Riley for valuable comments and also to Cicada films and Prof., Tony Ryan for work on the Baghdad Battery. We would like to dedicate this article to Jan Meering who taught at the Angmering School, West Sussex and all kids who went through our workshops there.
THE CREATIVE SCIENCE CENTRE
home | diary | whats on | CSC summary | latest news