The Discovery of C60,
Buckminsterfullerene & The Fullerenes

Introduction
C60, Buckminsterfullerene [1] the third allotropic form of carbon was
discovered in tiny quantities in 1985 by H. W. Kroto (Sussex Uni., UK) and
R. E. Smalley (Rice Uni., USA). In 1990 a method was developed to make C60
(and the family of carbon cage molecules - the fullerenes) in gram
quantities. Fullerene science has now become a rapidly growing field of
research [2]. This article briefly describes the fascinating experiments that first
uncovered the fullerenes and the subsequent techniques used to make bulk
quantities of them.
Cluster beam experiments
C60 and the fullerenes were discovered on a versatile and ingenious piece of
equipment called a cluster beam apparatus [1,2]. The beam experiments were
essentially very simple; an element (in this case carbon - graphite) was
rapidly heated (under vacuum or inert gas) by a high power laser, reaching
temperatures in excess of 10,000 °C - hotter than the surface of the Sun.
The vaporised products were then analysed using a sensitive mass
spectrometer. An additional refinement of the experiment was that the laser
vaporised products were rapidly cooled before being fed into the mass
spectrometer, this 'froze' out the reactivity of the various species
produced. Without this many of the species produced would rapidly go on to
form larger systems with their neighbouring vaporised atoms and molecules.
The technique therefore takes a sort of 'snap-shot' of the initial products
of the vaporisation.
The heart of the cluster beam apparatus was the mass spectrometer. This device separates out all the products in terms of their mass and displays the result in the form of a graph. This spectrometer makes the machine versatile and exquisitely sensitive. When carbon was analysed using this apparatus a whole range of structures (clusters of atoms) were observed, in fact the spectrum of carbon was one of the most interesting of all elements. Atoms, small molecules, large molecules, small particles, large particles and graphite fragments were all observed. At first sight it was obvious that a random mixture of products were produced by the laser vaporising the graphite. On closer inspection, certain sized clusters of atoms appear to be more abundant, stable and resistant to reaction than others. These 'magic' numbered species were shown to have the closed shell structures - the fullerenes - with C60, Buckminsterfullerene (the football molecule), usually being the most dominant [1,2].
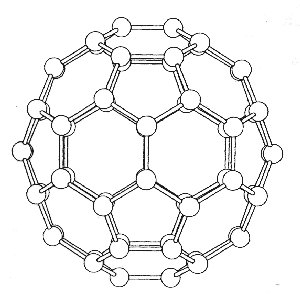
Analysis of the data suggested that the most reasonable solution was that the carbon molecules were forming closed cage structures. C60 has a particularly wonderful structure, that of a truncated icosahedron. The truncated icosahedron has 12 pentagon and 20 hexagon rings and has 60 vertices it is also the shape of the soccer ball football !
There was really only one problem in these fascinating and important experiments, and that was due to the amazing sensitivity of the spectrometer. You see, on the one hand this allowed the fullerenes to be first observed (under formation conditions that were probably far from favourable) but on the other hand it also meant that only tiny quantities were actually being produced at any one time. The machine was capable of detecting nanograms of C60. A rough calculation shows that even if one runs the cluster beam apparatus for ten years or so, - non stop - one would barely produce enough C60 to line the bottom of a test tube (perhaps only a few milligrams would be produced).
This amazing technology therefore puts us in a rather tantalising situation;
it allows us to make new discovers but then leaves us with the problem of
being able to make large enough amounts to be able to do anything with.
Therefore the promising new area of C60 science (for example the physical
and chemical properties) would have to wait until we could make large
quantities - at least on the milligram scale.
The carbon arc
The solution to this dilemma came about with the breakthrough made in 1990
by W. Krätschmer and D. R. Huffman (a German-American team [4]) and to a
certain extent the Sussex University team [2,5]) using apparatus that might
well have been available back in 1890 ! Unlike the expensive high-tech
cluster beam apparatus that discovered the fullerenes, the apparatus that
first produced gram quantities of C60 was incredibly simple. Bulk quantities
of C60 were first produced using a carbon arc. The technique works like this,
Two high purity carbon rods (roughly 5cm long and 0.5cm diameter) were supported so that their ends just touched. This rod system was mounted inside a glass bell-jar. The bell-jar was evacuated and filled with helium (or argon) to 100 Torr (roughly a seventh of an atmosphere pressure, 700 Torr = 1 atm). A large electrical current (20 volt at about 100 amps) was then passed through the rods, developing a bright arc-discharge between them. This was maintained for about 10 - 20 seconds during which time the arc sputters black soot like material throughout the jar. After letting the apparatus to cool down the bell-jar was opened up and the soot scrapped out.
The type of deposit found inside the bell-jar depends critically on the inert gas pressure. Under vacuum a hard, shiny brown graphitic layer was deposited which was difficult to remove. Introduction of only a small amount of inert gas dramatically changes the type of layers deposited. For fractions of a Torr of helium, the deposit settles as a fine jet-black powdery soot layer or film, which can be removed without difficulty. The soots remain jet black until the gas pressures reach c.a. 10 Torr, where the film develops a dark brown hue. On closer inspection these films appear to have a crystalline component, adding a slight sparkle to the dull soot layer. Similar results are obtained for argon, although the transition pressure is a little higher.
Providing the rods were fairly pure (better than a few % purity, ie. no
sulphur content) and that no air leaked into the jar during arcing, 5 - 10 %
of the soot produced in this arc treatment is actually C60. Its as simple as
that !
The fullerenes are soluble
The next step was to try and extract the fullerenes from the arc materials.
Adding toluene (or benzene, hexane, chloroform, carbon disulphide etc) to
the soot and leaving the resultant mixture to stand for a few hours, we find
that the fine suspension of soot particles will settle and the solvent will
have turned red (this was first done at Sussex 6 August 1990, [2,17]). Mass
spectrometry shows that the solution contains C60 and larger fullerenes.
Solvent extraction of the fullerenes is therefore possible. Analysis shows
that C60, C70 and traces of the larger fullerenes make up roughly 80, 20 and
less than 1 % of the isolated material respectively [5].
It is this solubility which allows the fullerenes to be separated
effectively by chromatography (see below) and enables chemical reactions to
be studied systematically and conveniently.
Improving extraction - soxhlet method
Improved fullerene yields were obtained using an ingenious device called a
Soxhlet extractor [5,6]. The soot was loaded into
a thimble (typically ca. 2 - 3 g of soot; 100 x 30 mm thimble) and placed
into the extractor. Hot solvent condenses and drops on to the soot,
dissolving the fullerenes. Eventually a siphon arrangement draws the
saturated solution away so that a fresh batch of solvent can further extract
the soot. In this way the maximum amount of soluble material can be
extracted. Because of the toxicity of benzene one generally uses chloroform
or toluene. Although fullerene solubility might be slightly lower in these
solvents it does not hinder the extraction process significantly because the
soot is washed many times. Extraction of c.a. 3 grams of soot takes about 2
- 3 hours, and is judged to be complete after the colour stops leaching from
the thimble (although small traces of the larger fullerene > C70 may well
take 10's hours to remove completely). Using this method 5 - 10 % of the
soot was found to be soluble, where the majority of the extract are
fullerenes. The extract solution can then be evaporated to give a
brown-black solid. This extract is washed in acetone to remove hydrocarbon
impurities which may be present from the solvents.
Chromatography
Having found that the extracted material consists of a mixture of molecules
their separation was achieved at Sussex by column chromatography [2,5,6,7].
A chromatography column (glass tube ca. 30 cm long x 1 cm diameter, glass sinter + tap) was filled with carbon granules (Elorite grade see [19]). The bottom tap was opened and toluene added until the level reached the top of the granules and no more was absorbed. The concentrated extract (ca. 30 mg in 100 ml of toluene) was then passed down the column. When all the extract was loaded onto the column, fresh toluene was applied. After roughly 10 minuets (from first applying the extract) the first coloured fractions should emerge from the column. This band is a beautiful magenta colour and consists of pure C60. Very soon after the first coloured fraction has finished, a second band appears that is red. This fraction is a mixture of C60 and C70 (the colour of the former is masked by the latter).
Using this technique, pure samples of C60 can easily be prepared. However
the red C70 fraction will still contain C60 and so further chromatographic
separation has to be carried out on these fractions to produce pure C70.
Chromatography of large amounts of extract (ca. 100 mg) also produced other
weakly coloured bands following the C70 fractions. These are due to higher
mass (larger) fullerenes present at very low concentrations (ie. see
reference [8] for examples of larger fullerenes greater than C70)
Further Purification
After repeated chromatography, solutions of relatively pure C60 and C70 can
be prepared (better than 95 % pure by spectroscopy). The solvent can be evaporated to
leave the solid fullerene. However, the dry solid still contains a
significant quantity of solvent trapped in the crystal lattice.
For example, IR (Infrared) spectroscopy of thin films of C60, show benzene
peaks when evaporated from this solvent. Whatever solvent one uses there is
always trapping in the crystal lattice (perhaps a few % of the mass). One
can substantially reduce this by baking the fullerenes at 550 K for several
hours under vacuum (less than 1/1000 Torr). However, defect-free samples of the
fullerenes can only be made by subliming the samples (i.e. heating at ca.
800 K and collecting the sublimate) and then heating for days at a constant
temperature (c.a. 600 K) under vacuum. In this way samples can be annealed
to produce material of high quality.
return to C60 page
return to 'Things to Make' page
References, notes and diagrams
Scientific articles and notes (articles = name, Journal, year, vol., page)
[1] H. W. Kroto, J. R. Heath, S. C. O'Brien, R. F. Curl, R. E. Smalley,
Nature, 1985, 318, 162.
[2] H. W. Kroto, C60: Buckminsterfullerene, The Celestial Sphere that Fell
to Earth,
Angewandte Chemie, 1992, 31, 111 - 246.
[3] J. P. Hare, Sussex University, C60 and Schools, Past Sixteen Science
Issues, Feb. 1996.
[4] W. Krätschmer, L. D. Lamb, K. Fostiropoulos, D. R. Huffman, Nature,
1990, 347, 354.
[5] R. Taylor, J. P. Hare, A. K. Abdul-Sada, H. W. Kroto, Journal of The
Chemical Society,
Chemical Communications, 1990, 1423.
[6] J. P. Hare, R. Taylor, H. W. Kroto, Chemical Physics Letters, 1991, 177,
394.
[7] A. D. Darwish, H. W. Kroto, R. Taylor, D. R. M. Walton, Improved
chromatographic
separation of C60 and C70,
Journal of the Chemical Society, Chemical Communications, 1994, 15 - 16.
[8] See articles in Physics and Chemistry of Fullerenes, Editor P. S.
Stephens,
Advanced Series in Fullerenes, Vol. 1, World Scientific Publications, 1993,
ISBN 981-02-1117-1.
[9] A vertical rod-block generator with a large volume (ca. 10 litre)
stainless steel chamber.
[10] R. E. Haufler, Materials Research Society, Symposium Proceedings, 29th
Nov. 1990.
[11] D. H. Parker, P. Worz, K. Chatterjee, K. R. Lykke, J. E. Hunt, M. J.
Pellin,
J. C. Hemmiger, D. M. Gruen, L. M. Stock, Journal of the American Chemical
Society, 1991, 113, 7499.
[12] A. S. Koch, K. C. Khemani, F. Wudl, Journal of Organic Chemistry, 1991,
56, 4543.
[13] G. Peters, M. Jansen, Angewandte Chemie, International (English)
Edition 1992, 31, 223.
[14] R. E. Haufler et al. Material Research Society, Symposium Proceedings,
1991, 206, 627 - 637.
[15] K. Yoshie, S. Kasuya, K. Eguchi, T. Yoshida, Applied Physics Letters,
1992, 61, 2782.
[16] W. A. Scrivens, J. M. Tour, Journal of Organic Chemistry, 1992, 57, 6932.
[17] J. P. Hare, PhD Thesis, University of Sussex, Chemistry and Molecular
Sciences, 1993.
Carbon rod suppliers
[18] Le Carbon, South Street, Portslade, Brighton, East Sussex, BN41 2LX.
Tel. 01273 415701, Fax. 01273 415673. Purity 6 rods,
4.6 mm diameter x 303 mm long, ca. £ 200 for 100).
Agar Aids Ltd, 66a Cambridge Road, Stansted, Essex, CM24 8DA
(for small high purity rods, Type E340, 5 mm diameter x 50 mm
ca. £10-00 for 10).
Chromatography suppliers
[19] Toluene need not be high purity.
Fissons may sell an appropriate column
Carbon granules from;
Norite Ltd, ELORITE grade, carbon granules, sample number A-8160, Norit
N. V., Postbus 105, 3800 AC, Amersfoot, The Netherlands.
AC welding power supplies
[20] RS components, Turboweld 8, SN 623-271, page 3-3125 Radio Spares Catalogue
or borrow one , have one donated, or steal one from a builder, ironmonger
or garage.
or try a fully charged car battery.
home | diary | whats on | CSC summary | latest news